
|
Elecnano4 - 7th ECHEMSParis, France23 - 26 May 2011 |
|
Core-Shell Dendrimer-Encapsulated Nanoparticles: Theory, Synthesis, Characterization, and Electrocatalysis
Richard M. Crooks, Emily V. Carino, Graeme Henkelman, Hyun-You Kim,
David F. Yancey, and Liang Zhang
Department of Chemistry and Biochemistry, The University of Texas at Austin
1 University Station, A5300, Austin, TX78712-0165USAOne approach for designing improved nanoparticle electrocatalysts involves the use of first-principles calculations, such as density functional theory (DFT), to predict the structural properties of efficient, new materials. As these types of calculations have begun to emerge, however, it has become increasingly clear that there are few or no experimental models available to test them. Dendrimer-encapsulated nanoparticles (DENs)(1) provide an opportunity to meet this need, because their size, composition, and structure can be controlled and because they have a size that is compatible with DFT calculations (< 100 atoms). DENs are synthesized by complexing metal ions with interior tertiary amines of poly(amidoamine) (PAMAM) dendrimers, followed by chemical reduction. By controlling the metal-ion-to-dendrimer ratio, the size of DENs can be controlled. In addition, bimetallic DENs have been prepared by complexing and chemically reducing different metals either simultaneously, which usually yields alloys, or sequentially, which can lead to core/shell structures. This chemical reduction method has proven effective, but here we will present a new approach for synthesizing core-shell DENs, which is based on the electrochemical method of underpotential deposition (UPD), that provides a means for exerting a higher level of control over shell structure.
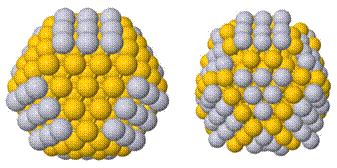 Cu UPD onto Au[2] or Pt[3] DENs, having sizes ranging from 1.2 to 2.0 nm, results in core-shell nanoparticles. Our present understanding of both full- and partial-shell structures will be discussed from both experimental and theoretical perspectives. As illustrated schematically in the figure, both full and partial Cu shells on Au@Cu DENs can be replaced with Pt via a galvanic exchange reaction.2 Both in-situ and ex-situ X-ray absorption spectroscopy (XAS) has been used to characterize these materials. More traditional characterization methods, such as cyclic voltammetry, UV-vis spectroscopy, and high-resolution electron microscopy also provide insights into the structure of these core@shell DENs. The electrocatalytic properties of these materials for the oxygen reduction reaction (ORR) and the hydrogen evolution reaction (HER) will be discussed as a function of the identity of the core metal and the identity and coverage of the shell. These experimental results will be correlated to preliminary DFT calculations.
Cu UPD onto Au[2] or Pt[3] DENs, having sizes ranging from 1.2 to 2.0 nm, results in core-shell nanoparticles. Our present understanding of both full- and partial-shell structures will be discussed from both experimental and theoretical perspectives. As illustrated schematically in the figure, both full and partial Cu shells on Au@Cu DENs can be replaced with Pt via a galvanic exchange reaction.2 Both in-situ and ex-situ X-ray absorption spectroscopy (XAS) has been used to characterize these materials. More traditional characterization methods, such as cyclic voltammetry, UV-vis spectroscopy, and high-resolution electron microscopy also provide insights into the structure of these core@shell DENs. The electrocatalytic properties of these materials for the oxygen reduction reaction (ORR) and the hydrogen evolution reaction (HER) will be discussed as a function of the identity of the core metal and the identity and coverage of the shell. These experimental results will be correlated to preliminary DFT calculations.
References:
1 Scott, R. W. J.; Wilson, O. M.; Crooks, R. M. J. Phys. Chem. B 2005, 109, 692-704.
2 Yancey, D. F.; Carino, E. V.; Crooks, R. M. J. Am. Chem. Soc. 2010, 132, 10988-10989.
3 Carino, E. V.; Crooks, R. M. Langmuir, 2011 (published on the ACS website).










